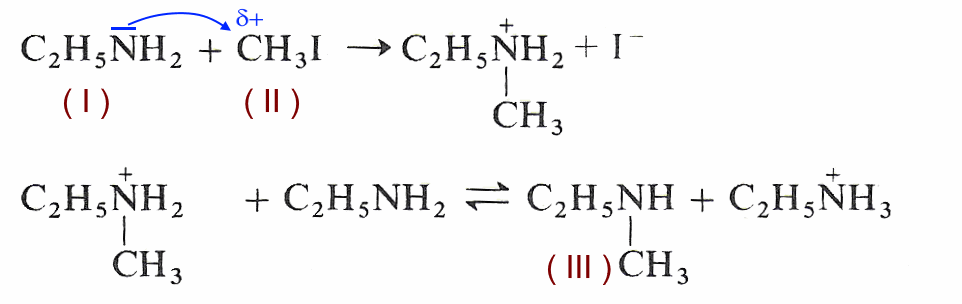Ethylamine Organic Base Molecule. 3D Rendering. Atoms are Represented As Spheres with Conventional Color Coding: Hydrogen White. Stock Illustration - Illustration of color, chemistry: 187971239

Relative Basicity of Ammonia, Ethylamine & Phenylamine (7.6.3) | CIE A Level Chemistry Revision Notes 2019 | Save My Exams
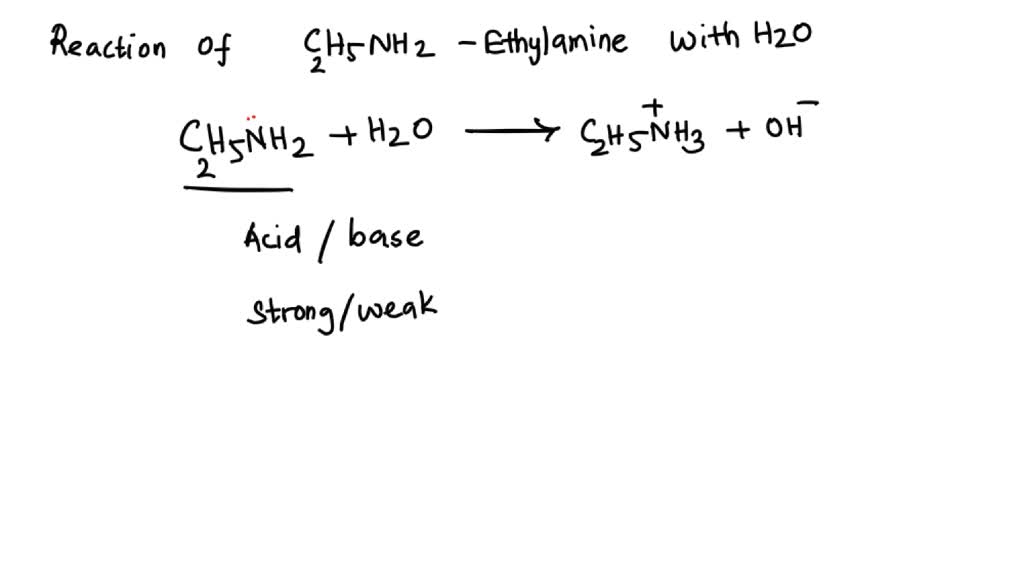
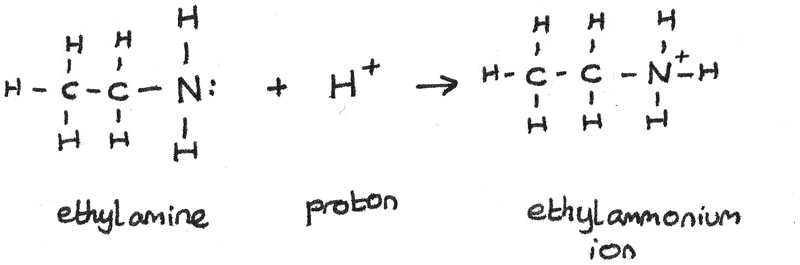
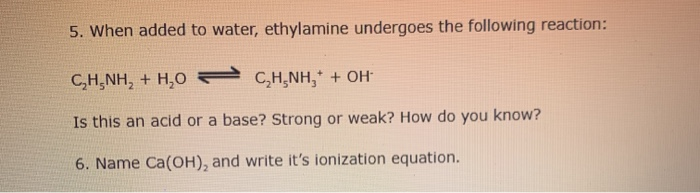
SOLVED: 5. When added to water, ethylamine undergoes the following reaction: C2H5NH2 + H2O C2H5NH3+ + OH- Is ethylamine an acid or a base in this reaction? Strong or weak? How do

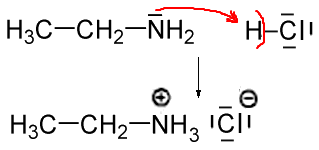
Acid-base chemistry of aliphatic amines weak bases pKb Kb values why stronger than aomatic amines reactions with acids primary secondary tertiary balanced neutralisation equations organic nitrogen compounds organonitrogen molecules advanced A level

Ethylamine organic base molecule. Stylized skeletal formula (chemical structure): Atoms are shown as color-coded circles: hydrogen (beige), carbon (grey), nitrogen (blue Stock Photo - Alamy

SOLVED:Which member of each pair is the stronger base? a. ethylamine or aniline b. ethylamine or ethoxide ion c. phenolate ion or ethoxide ion d. phenolate ion or acetate ion